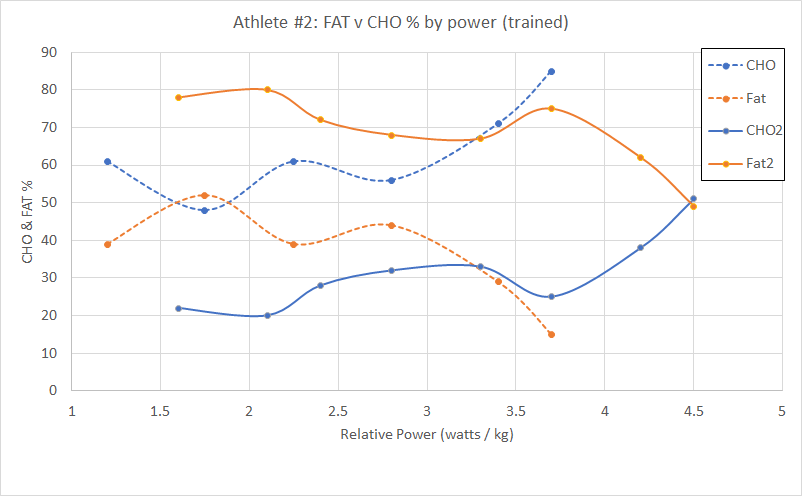
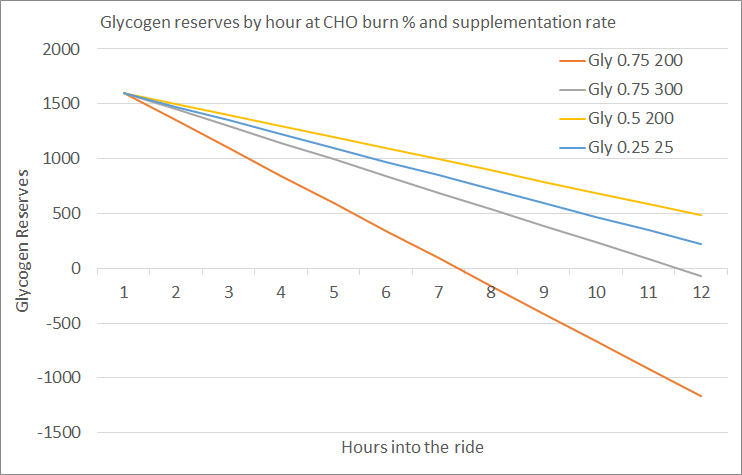
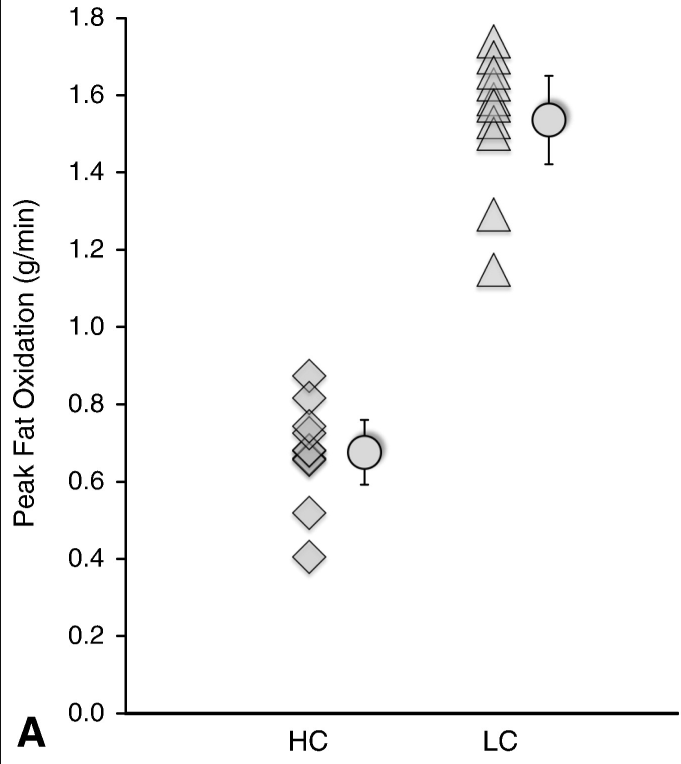
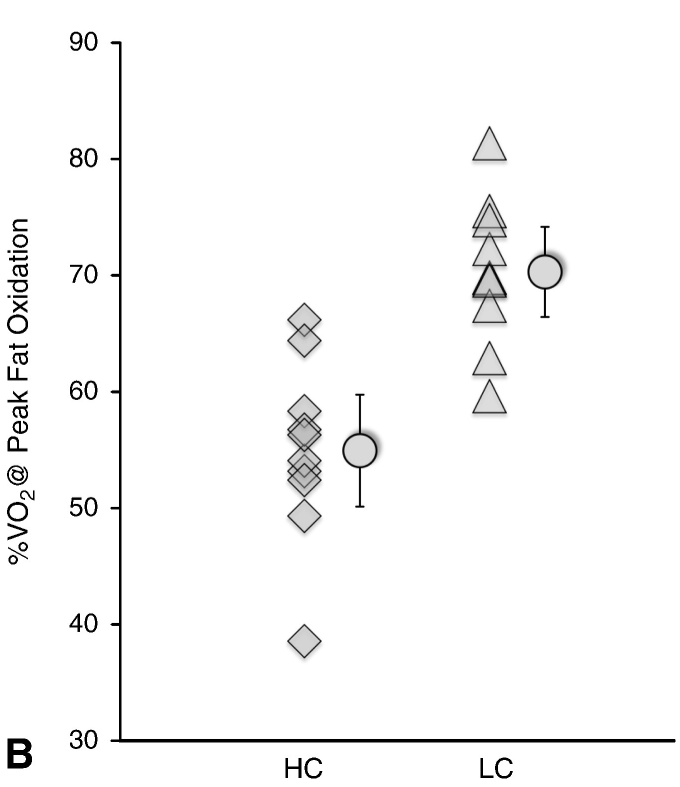
Please read the previous posts if you haven’t seen them before…
We are moving closer to the post where I hope to give useful advice on the different tactics you might use to improve fueling or lose some weight. Or perhaps both.
This was going to be a short post as I was having trouble coming up with a good way to talk about hunger, but I came across some new (to me) information that I hope will be informative.
Hunger
Hunger lies at the intersection of energy balance, brain function, psychology, and group dynamics. Oh, and evolutionary biology. That makes it complex and hard to understand, and therefore not very amenable to easy explanations or simplification. It’s much more complex than – for example – how blood glucose is controlled. I’m therefore going to have to simplify quite a lot, and there will be a number of areas where it’s just not clear (to me) what is going on. That said, let’s get started.
The evolutionary purpose of hunger is to drive us to eat in ways that maintain our energy stores – and in particular, our fat stores – at a certain level. Or perhaps within a certain range. What factors could set the low and high limits of that range? (note 1).
Let’s start at the lower end – what drives the lower end of the fat storage range?
If we have too little stored energy, we won’t be able to survive when food becomes scarce.
If we are female, we need extra stored energy to be able to build and feed a child.
And at the upper range?
If we have too much stored energy, we may not be able to move around effectively, which could compromise our survival.
There may also be impact based upon climate, but I’m going to ignore that for this discussion.
Another way to describe the range is “enough fat, but not too much fat”. Where “enough” and “too much” have definitions that are a bit squishy. But you get the idea…
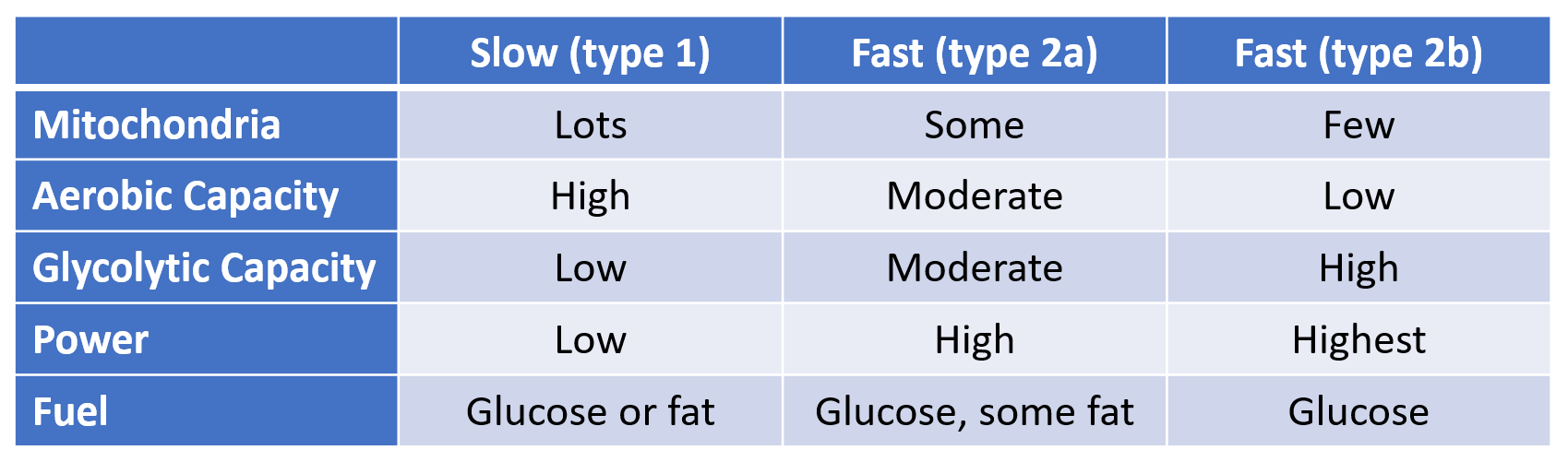
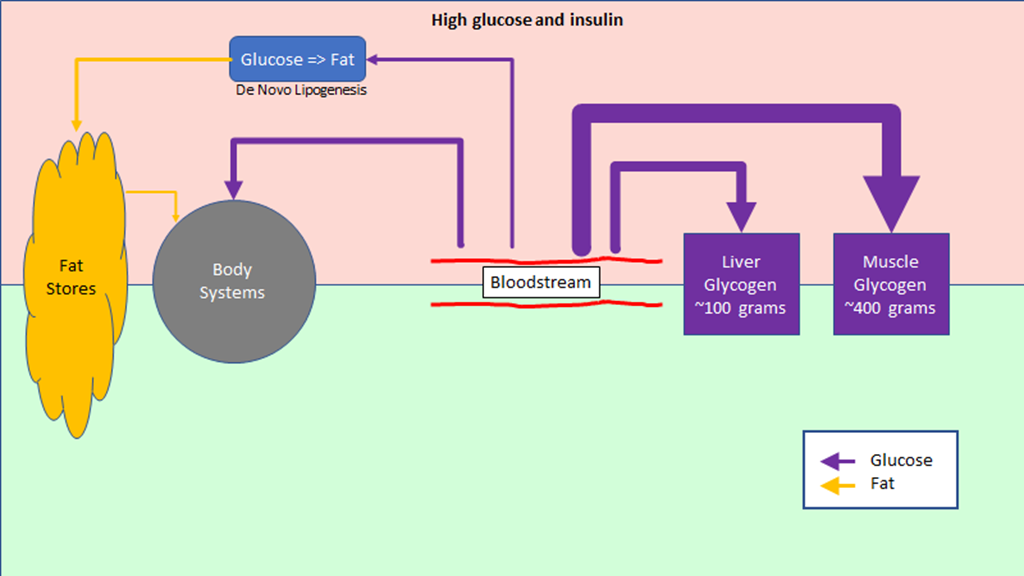
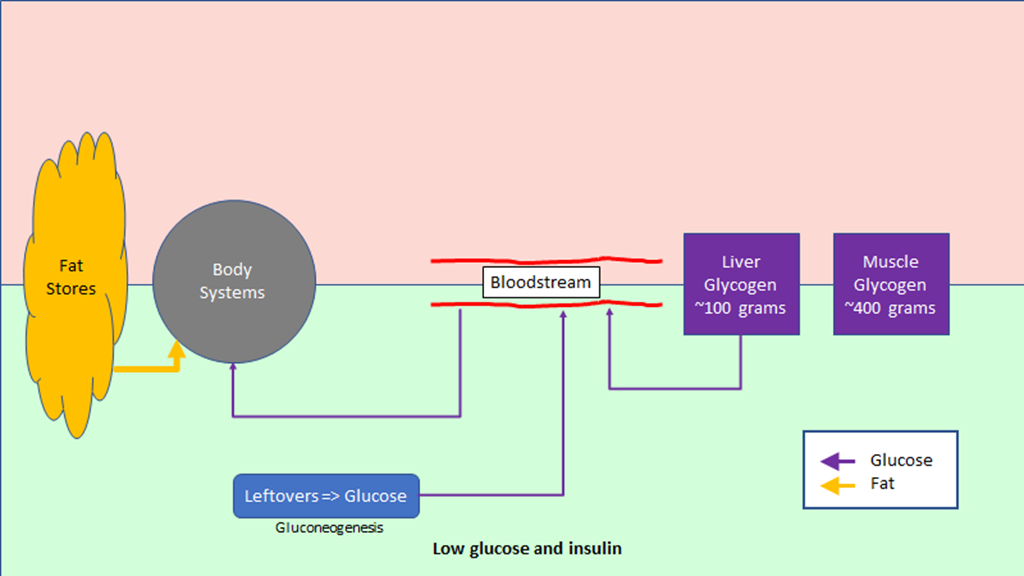
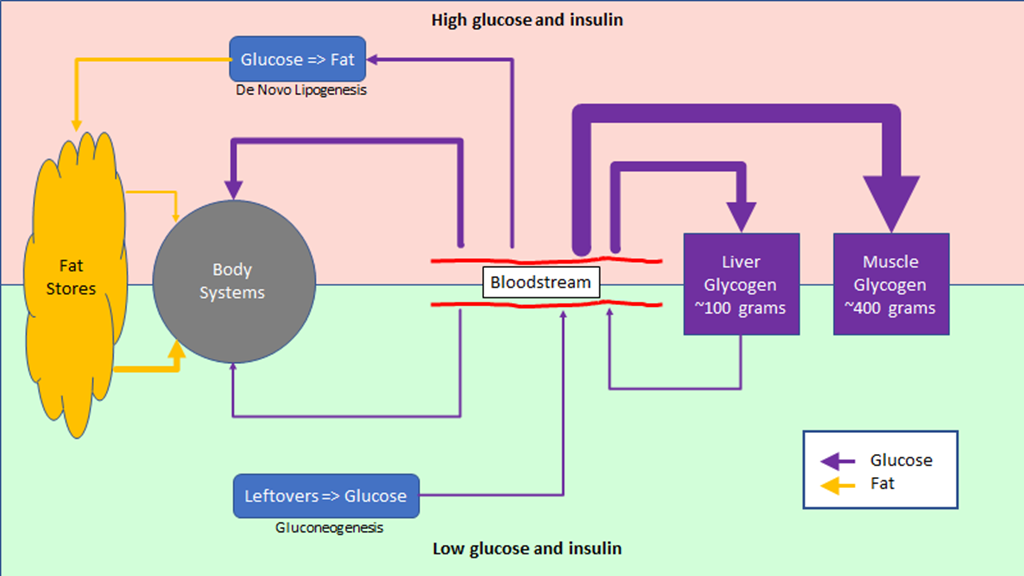
The regulation of hunger – and therefore the regulation of energy intake – is driven by two hormones, leptin and ghrelin. To oversimplify things:
Leptin is produced by fat cells, and serves to reduce hunger. Generally speaking, the larger the fat storage, the higher the leptin levels will be. You can think of leptin levels like the gas gauge on a car; if your fat tank is empty, leptin reads low, if you fat tank is full, leptin reads high.
Ghrelin is produced by the stomach, and serves to enhance hunger. It’s a shorter term signal.
Based on a simple understanding of how the hormones work, we would expect that Ghrelin levels would be proportional to how long it was since we ate; the longer we went without food, the more hungry we would be.
Here’s a graph of Ghrelin values over a typical day:

(note 2)
The solid line is the average while the dotted lines show the upper and lower range.
That’s not what I expected. Ghrelin levels are lowest right when we get up, which is when we have gone the longest without eating and would therefore expect them to be highest. It turns out that ghrelin levels have a few somewhat interesting features:
They are adaptive based upon when we usually eat and our circadian rhythms.
They increase when we start to eat. This is familiar to most of us; not being really hungry but finding out that as we smell dinner or start eating, we are suddenly hungry.
There’s another strange feature of ghrelin related to fasting. Here’s a study (note 3) that looked at ghrelin levels over an 84 hour fast:
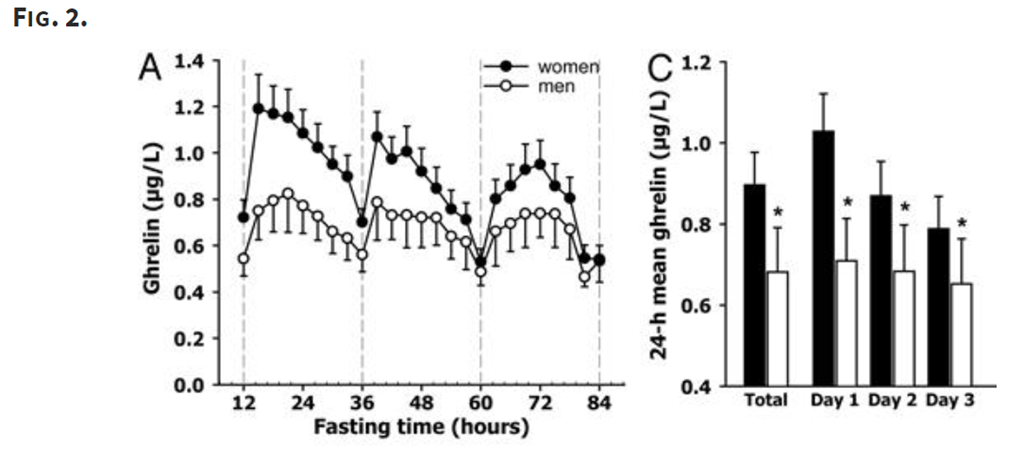
That’s just weird. The mean ghrelin levels decrease from day do day, which means you are actually quite a bit less hungry on day three of fast than you are in day one. While weird, this is pretty well established by research.
Also note that there seems to be a greater reduction for women, for reasons that are not well understood.
Why our bodies behave this way is not really known. The best theory I know is one from an evolutionary standpoint; while it is good to be hungry if food is available, it can quickly become counter-productive if food is scarce.
Moving onto leptin, what do leptin levels look like? (note 4):
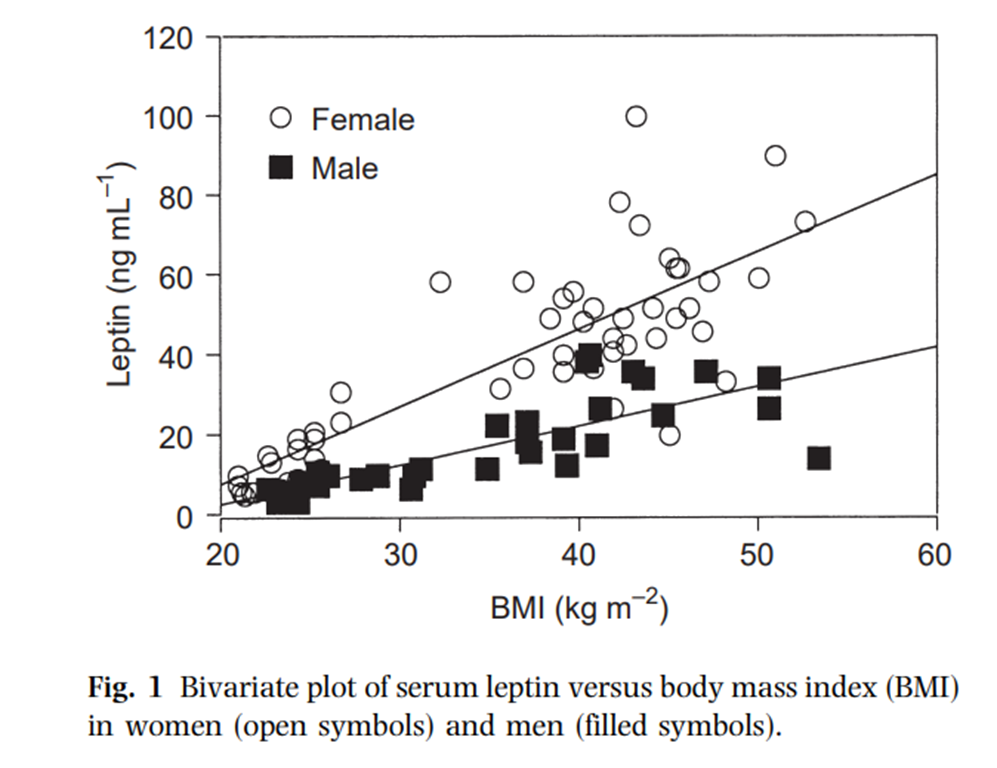
That is what we generally expect – at least there seems to be a decent linear relationship between BMI and leptin levels. It’s a bit messy, probably because BMI does not correlate perfectly to fat mass, and likely because of individual variations as well. The differences between male and female leptin levels are asserted to be caused by a) women having more fat mass at a given BMI and b) women having more of the kind of fat cells that produce more leptin than men, though I don’t think the question is truly settled.
Disfunction
Leptin is supposed to inhibit significant weight gain; if you gain excess fat, your leptin levels rise, your hunger drops, and you lose fat mass until your leptin levels drop. And that seems to work for some people, especially those who are young, choose their parents well, and male. But it’s pretty obvious it does not work well in a lot of cases.
Is there anything known about the disfunction? Well, a bit…
Leptin and ghrelin levels based on types of food
I found a very nice experiment (note 5) that looks into leptin and ghrelin response based on different kinds of food. Take a group of people, have them fast overnight, and then give them one of three drinks:
500 calories with 80%/10%/10% from carbs, fat, and protein
500 calories with 10%/80%/10% from carbs, fat, and protein
500 calories with 10%/10%/80% from carbs, fat, and protein
In other words, a carb-heavy (glucose-heavy), fat-heavy, and protein-heavy drink (note 6). This is done in what is called a “crossover” study, which means that each subject had all three drinks on different days.
You sample their blood before they have the drink, and then every 30 minutes afterwards, and measure a bunch of different things. Based on how ghrelin works, I would expect that eating would suppress the ghrelin levels and then over time, they would rebound to their previous levels.
What happens?
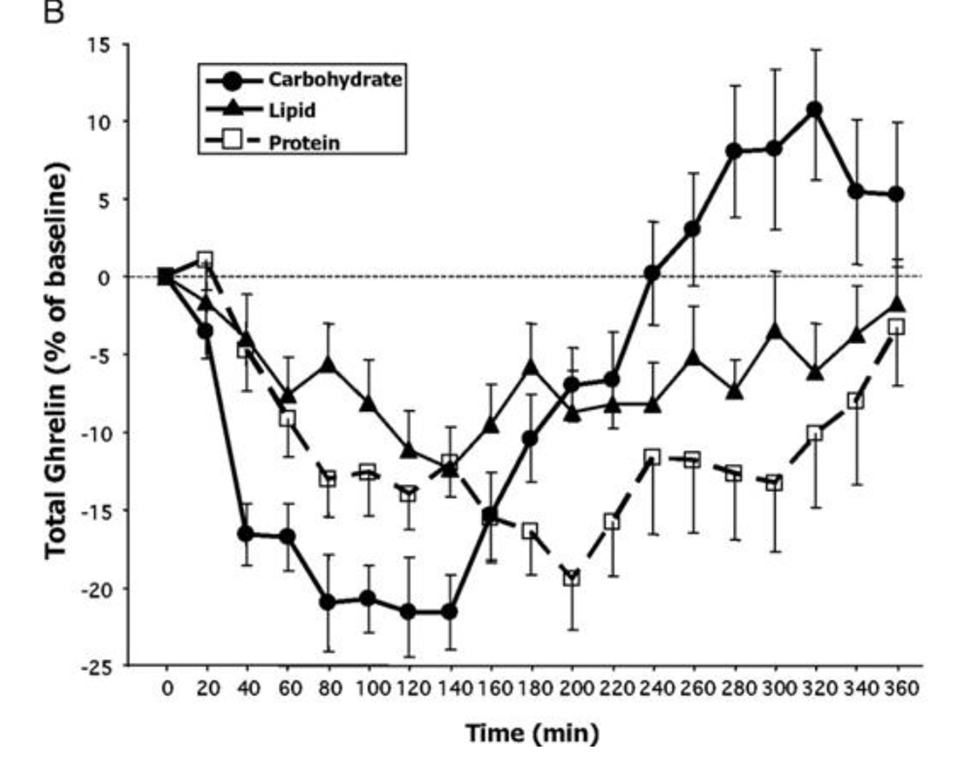
All three produce a significant suppression of ghrelin production, but carbohydrate produces the biggest reduction. Interestingly, however, after about two hours the carbohydrate ghrelin level goes shooting up and after 3 hours it is higher than the fat or protein curves and soon after becomes higher than the initial ghrelin level.
Or, to put this another way, five hours after eating 500 calories of mostly glucose we would be *more hungry* than we were at the start.
The authors write, “Our finding of a rebound of total and especially acyl-ghrelin above baseline after high-carbohydrate meals could provide some physiological basis for claims made by low-carbohydrate diet advocates that ingesting carbohydrates prompts an early hunger rebound”.
Indeed.
They did measure subjective appetite which showed no effect, though unfortunately they had technical difficulties with the appetite reporting system and that data was therefore not published.
I’d also like to note that there are many experiments that measure satiety (the inverse of hunger) and show that carbohydrates lead to more satiety than fats or protein. And they do, if you only measure them for 2-3 hours.
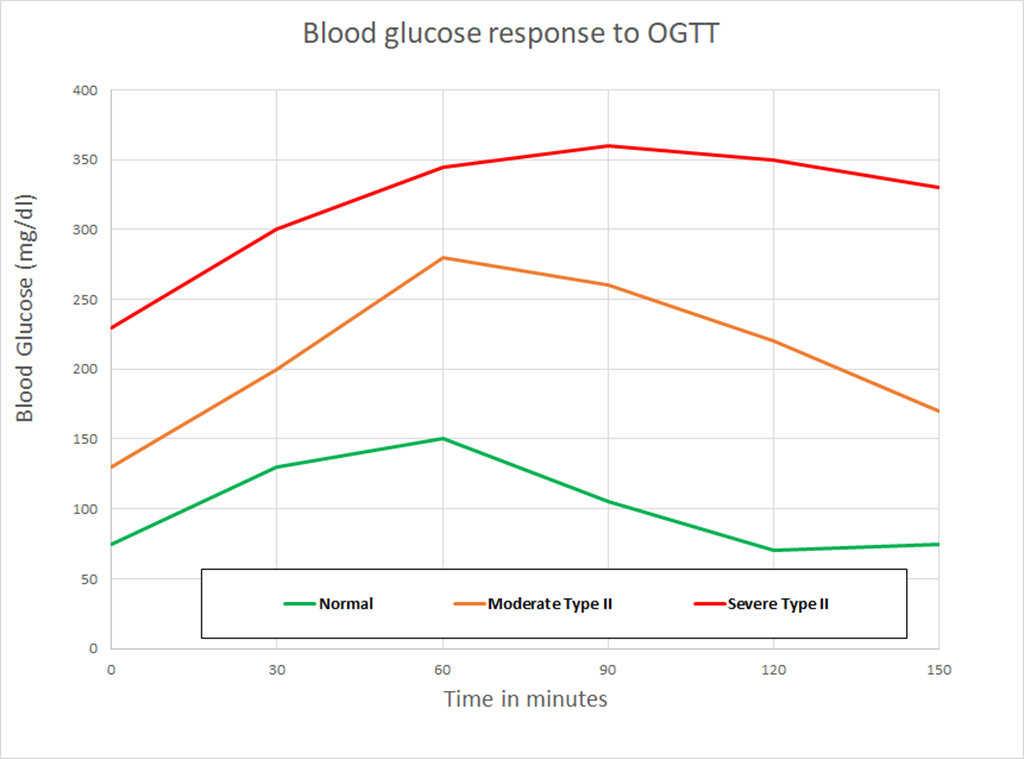
The experiment also measured blood glucose over time:
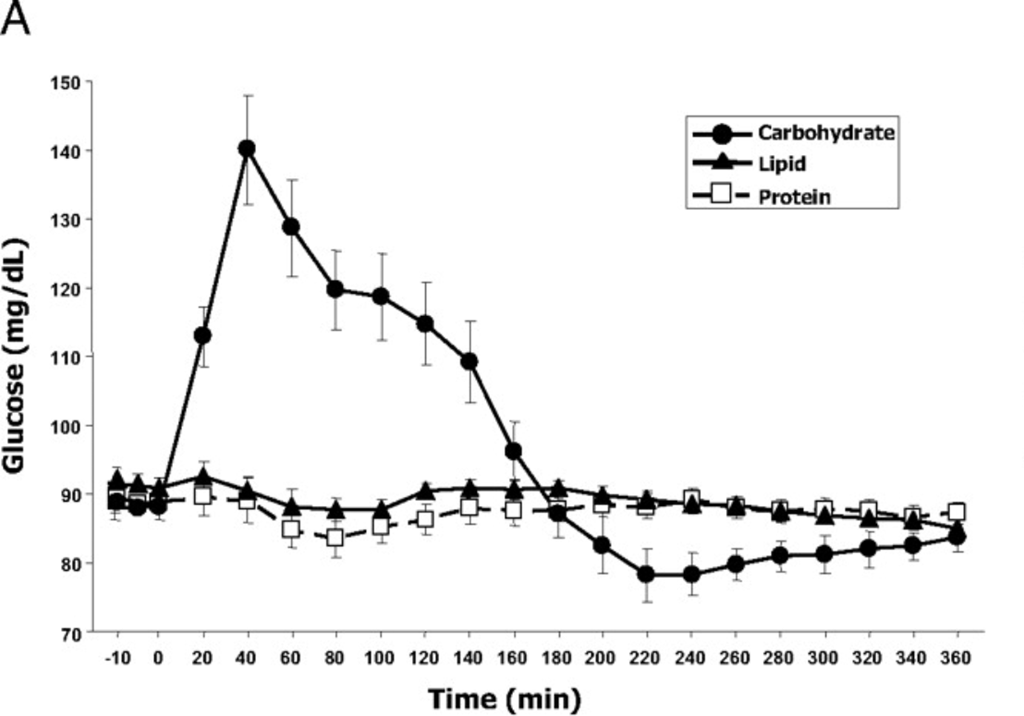
If I eyeball the two charts, it looks like ghrelin production starts to go up steeply about the time blood glucose drops quickly at 140 minutes, and is highest when the blood glucose is the lowest. The pattern here matches what is known as either “reactive hypoglycemia” or “postprandial hypoglycemia” – basically the blood glucose drops below initial levels a few hours after eating.
It’s important to note that the drinks were dominated by a specific macro and drinks with mixed macros may show unexpected results. Though 500 calories is not really that much and these were consumed in a fasted state, and as we know that is the time when the body is best able to handle a lot of glucose.
You can find the leptin chart in the paper if you’d like to see it; there were small drops over time but nothing very striking.
Fructose vs Glucose
Is there a difference between fructose and glucose? Let’s look at another experiment (note 7).
In this experiment, we take 12 normal-weight women and feed them three meals containing 55%, 30%, and 15% of carbohydrate/fat/protein and take blood samples every 30-60 minutes.
Of the 55% of the calories that come from carbs, 30% either comes from a fructose-sweetened or glucose-sweetened beverage. Take a normal meal pattern and make the carbs either fructose heavy or glucose heavy. And sample their blood periodically:
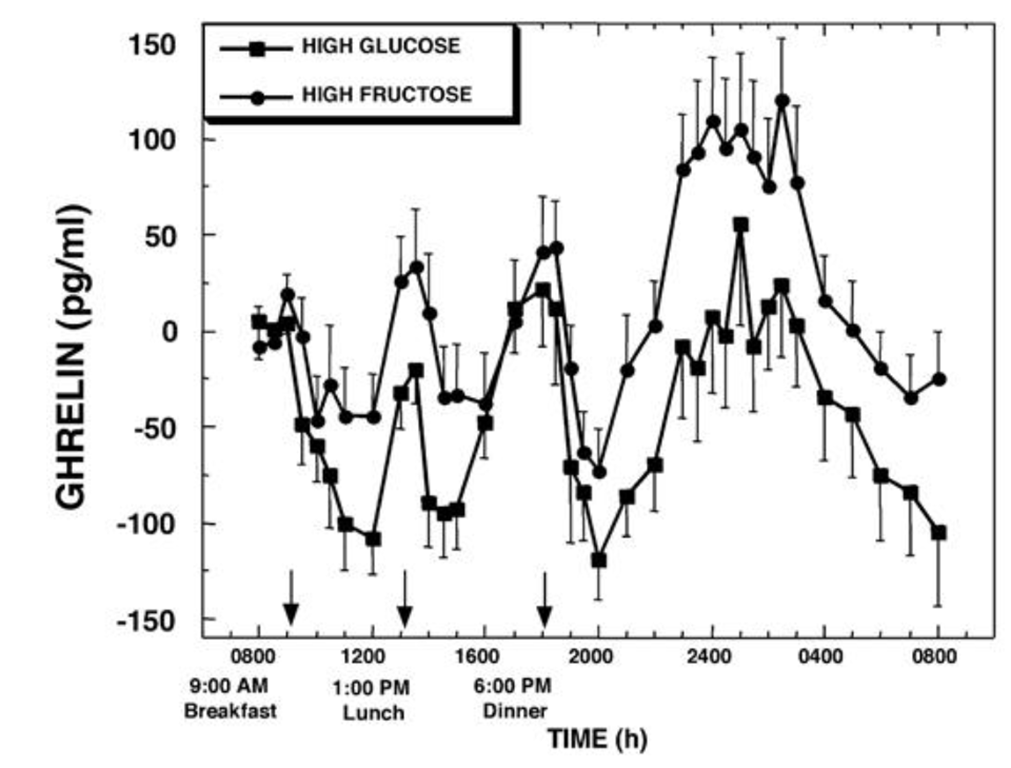
Wow. Lunch and dinner show large spikes in ghrelin for both drinks, but the late-night spike of the fructose drink is much higher. Also notice the difference in levels at 8am the next day; the level of ghrelin in those who had glucose is quite low, but it’s pretty high for those who had fructose.
Fructose gives us bigger positive ghrelin peak than glucose.
They also measured blood glucose levels:
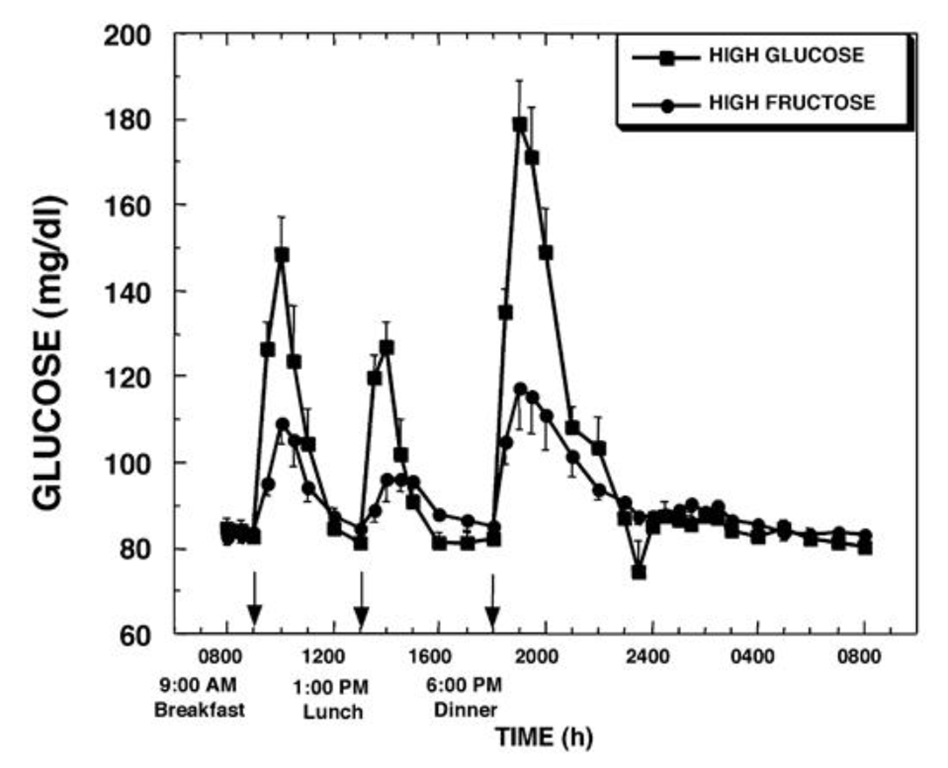
Based on what we know about glucose and fructose metabolism, that is what we would expect; because the fructose is processed in the liver, there is much less glucose. We would expect that the liver processes the fructose to triglycerides. Is there data to support that?
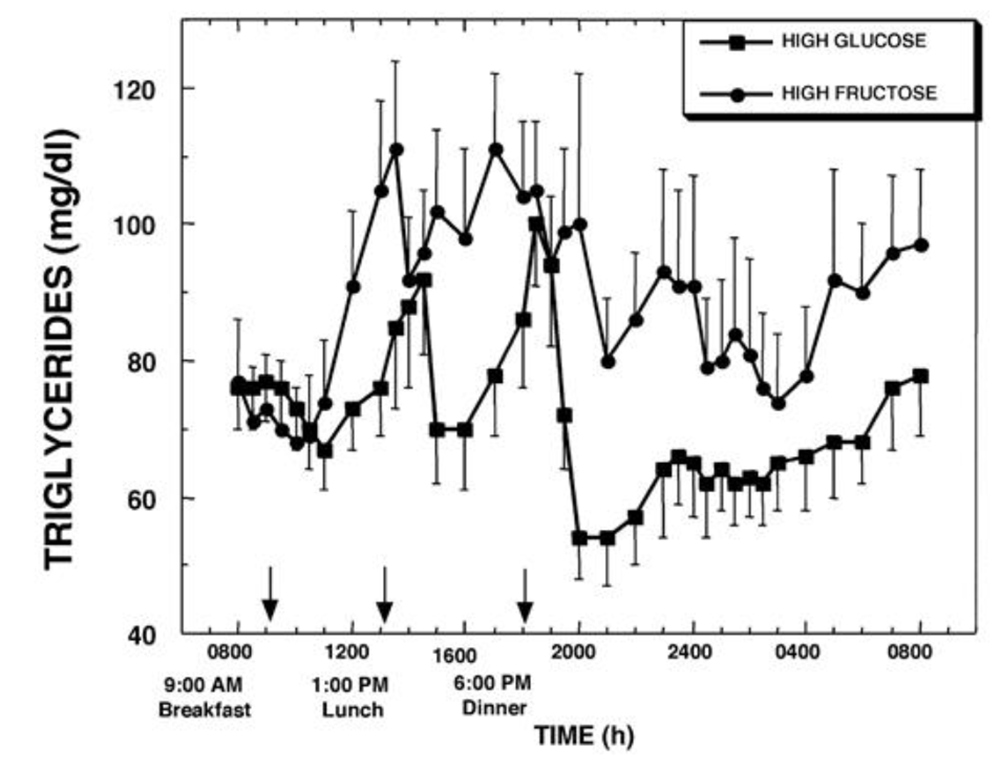
Yep. Vastly higher triglyceride levels show the fructose being converted to fat and released into the bloodstream, and those levels persist through the night.
They also measured leptin levels:
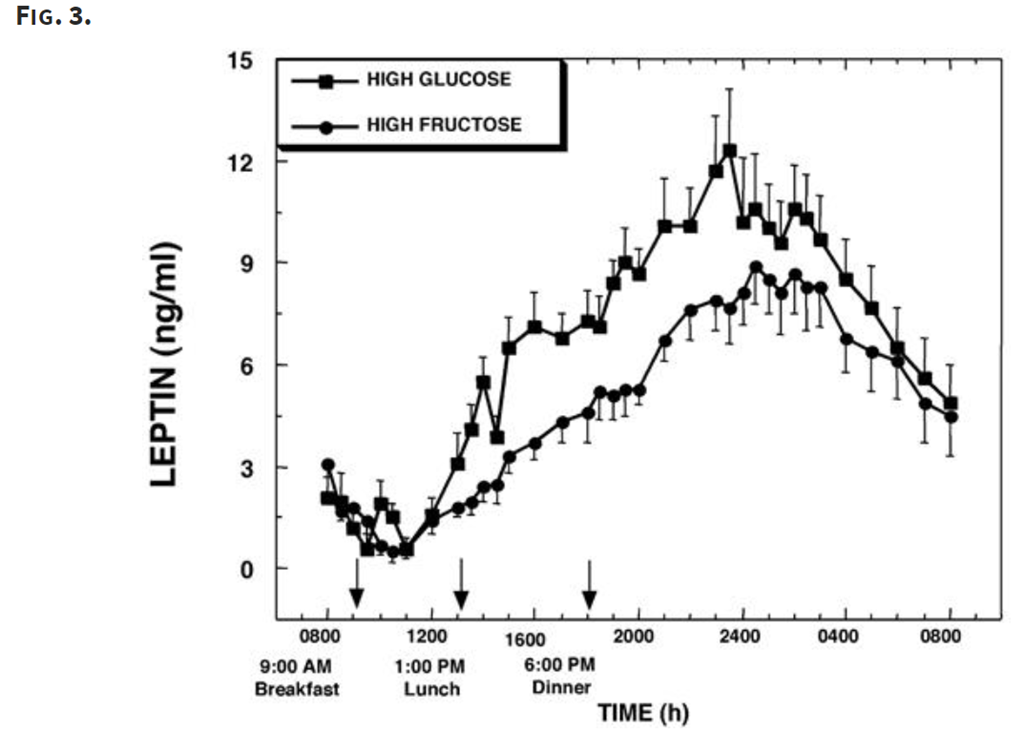
Leptin levels rose much less for the high-fructose meal, which means there was less inhibition of appetite.
Overall, fructose led to a greater increase in ghrelin (higher appetite) and a lesser increase in leptin (less appetite suppression).
So, carbohydrates in general aren’t great, and fructose is worse.
The best theory I’ve seen around why fructose behaves so differently is that significant fructose supplies were rare in historical times, and therefore it was advantageous for humans to eat as much as possible when they found them. That sounds reasonable, though like many studies in evolutionary biology they are hard to support.
Leptin resistance
The mystery of why leptin isn’t behaving as we would expect – why people still eat a lot even with high leptin levels – has been labeled “leptin resistance”, by analogy with insulin resistance; the idea is that for some reason the brain is not sensitive to the levels of leptin.
Whether there is actual resistance or whether there are other factors that are overpowering the leptin signal is not clear.
There are a number of theories round what is actually happening. Among them are:
There is a defect in transporting leptin from the bloodstream into brain cells across the blood/brain barrier.
The cells within the brain become less sensitive to leptin.
Dietary fructose leading to elevated triglycerides reducing leptin transport across the blood/brain barrier.
Here’s two papers if you want more information (note 8) (note 9).
Dopamine
I’m including this section for completeness. There is some research on how dopamine is affected by sugar ingestion, and while I think there something going on there that partially explains why sugar is addictive – at least for some people – I’m not confident enough in my understanding and the quality of the research to have much to offer.
I do offer a few papers:
Hunger Summary
The main points from the preceding section on hunger:
- Carbs – and especially fructose – seem to interfere with the hunger control system.
- Hunger is not directly related to how long it’s been since you ate; it has a daily rhythm and decreases when fasting.
Energy balance and weight loss
If you have read official guidelines and advice about weight loss, you can generally boil them down to one bit of advice:
“Eat less and move more”
This is often summed up as the “Calories in / Calories out” (CICO) model; eat less means fewer calories in, move more means more calories out, and the result will be weight loss.
It is also typical to see appeals toward the Laws of Thermodynamics. The more rabid adherents to the model treat it as if they have discovered one of the deep secrets of the universe.
If you have read the earlier posts and have learned anything about biochemistry, I’m sincerely hoping that you suspect that the reality might just be a *tiny* bit more complicated than the simple world of “eat less and move more”. Especially since that advice fails for a large number of people, at least for the long term.
There is truth to the CICO model in one sense, if you are losing weight you are burning more calories than you are taking in. And vice versa. But that’s the result, not the driver; the driver is the biochemistry at work.
The key to understand how things really work – and why CICO isn’t very useful in many cases – is related to how the body responds to a reduction in food intake. The body essentially has three options to balance things out:
- It can burn stored fat.
- It can tear down muscles (ie “lean mass”) and burn that.
- It can reduce its energy use.
We are hoping that it would do #1 – after all, the whole point of the fat storage system is to provide an energy reserve when food is scarce. But remembering the earlier posts, there are a couple of things that can get in the way of that. First off, we need to be good at burning fat in general. And second, we need to be in a hormonal state where fat burning is possible.
If either of those aren’t true – or are true only to a limited extent – then we are stuck with tearing down muscle and reducing energy use. And, in fact, that is what we see in a lot of diet studies; people will lose lean mass – sometimes a significant amount – and people report being cold, tired, and hungry all the time.
And they don’t really lose all that much weight.
If we can get rid of the conditions that are blocking fat metabolism – and, since we are athletes, up the amount of fat we burn when exercising – then the body should naturally start burning more stored fat, and we should lose weight. Or, to put it another way, we are going to focus on the fat burning side of the house.
This post is already quite long so I’ve tried to limit the detail in this section; if you want more I highly recommend Peter Attia’s post on fat flux.
Summary
There is a lot more that I could write about WRT hunger and energy balance, but I think this is enough for now.
Our strategy is going to make dietary modifications to reduce hunger and improve our ability to use fat to generate energy while exercising, with a goal to lose weight/improve our ability to perform in long events.
Which takes us to the tactics portion of the series. What do I think you should actually *do* to implement these strategies, so that you can – with any luck – see the benefits that I’m talking about.
That will be post #6. When I get that done, I’m planning on doing at least one post to cover any questions.
Post #6: Recommendations
Notes:
- I’ve tried to base this section on what I’ve learned about evolutionary pressure, fat stores, and hunger.
- From Jason Fung’s excellent discussion on hunger and fasting here.
- Fasting unmasks a strong inverse association between ghrelin and cortisol in serum: studies in obese and normal-weight subjects
- Mechanisms behind gender differences in circulating leptin levels. This result is widely replicated in other studies.
- Acyl and Total Ghrelin Are Suppressed Strongly by Ingested Proteins, Weakly by Lipids, and Biphasically by Carbohydrates
- The beverages were mostly composed of a glucose beverage, whey protein/nonfat milk, and heavy whipping cream for the carb/protein/fat drinks.
- Dietary Fructose Reduces Circulating Insulin and Leptin, Attenuates Postprandial Suppression of Ghrelin, and Increases Triglycerides in Women
- Leptin resistance: a prediposing factor for diet-induced obesity
- Mechanisms of Leptin Action and Leptin Resistance
- MarksDailyApple, by Mark Sisson’s. Mark is an advocate for a way of eating named “Primal”.